
The MiniMed™ 780G system is tested in trials and the real-world.
Proven clinical performance and economic value
Publications with MiniMed™ 780G system listed in PubMed
Randomised controlled trials
Health economics analyses
Real world evidence based on
85%
Of users achieve glycaemic targets1,2 with the support of the MiniMed™ 780G system3,
Better glycaemic outcomes
Real-world evidence from 101,629 users revealed that the MiniMed™ 780G system provides consistent outcomes in a diverse user population, in terms of ages, gender, geographies, and pre-automation glycemic control, and helps 85% of users to achieve the internationally recommended glycaemic targets.1-3,
MiniMed™ 780G system
Helps to achieve the HbA1c goal of 7.0%1 compared to other therapies.3,4
Helps to achieve the Time in Range goal of 70%2 compared to other therapies.3,4
Better glycaemic outcomes10,
less complications11
Improved glycaemic outcomes in terms of reduced HbA1c is associated with lower diabetic complication risks11
Original figure from Skyler 199611
With MiniMed™ 780G system, a 1.4% reduction in HbA1c is achieved compared to standard care10, projected to lead reduced complication incidences:12,
Less renal complications
Less eye complications
Less neuropathy complications
Less cardiovascular complications & stroke
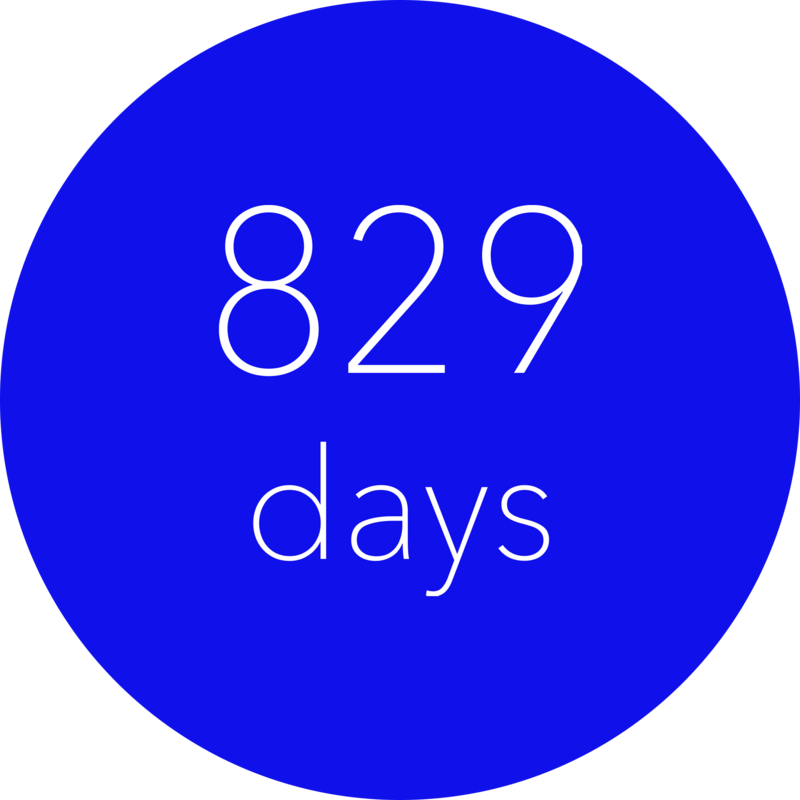
Gain up to 3 additional years free from diabetes-related complications12,
The MiniMed™ 780G system helps reduce the burden of type 1 diabetes by delaying costly diabetes-related complications and adding up to 3 more complication-free years compared to MDI + isCGM

Better glycaemic outcomes10,
less complications11,
more cost effective12,13
Did you know?
More than 50% of the direct healthcare cost for diabetes, are the costs of treating diabetes-related complications?14
MiniMed™ 780G system can help addressing this significant burden and offers greater quality-adjusted life expectancy12,



MiniMed™ 780G system: a worthwhile investment
Use of the MiniMed™ 780G system can lead to better glycaemic outcomes in people living with type 1 diabetes, leading to reduction in costly diabetes related complications and thus reducing treatment costs over long term.
A Medtronic Diabetes expert is available to explore this worthwhile investment with you
- ADA Guidelines of A1C<7% - ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic Targets: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- International Consensus on TIR>70% - Battelino T, Danne T, Bergenstal RM, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. doi:10.2337/dci19-0028
- Choudhary P, Arrieta A, van den Heuvel T, Castañeda J, Smaniotto V, Cohen O. Celebrating the Data from 100,000 Real-World Users of the MiniMed™ 780G System in Europe, Middle East, and Africa Collected Over 3 Years: From Data to Clinical Evidence. Diabetes Technol Ther. 2024;26(S3):32-37. doi:10.1089/dia.2023.0433
- Carlson AL, Sherr JL, Shulman DI, et al. Safety and Glycemic Outcomes During the MiniMed™ Advanced Hybrid Closed-Loop System Pivotal Trial in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther. 2022;24(3):178-189. doi:10.1089/dia.2021.0319
- Deshmukh H, Wilmot EG, Gregory R, et al. Effect of Flash Glucose Monitoring on Glycemic Control, Hypoglycemia, Diabetes-Related Distress, and Resource Utilization in the Association of British Clinical Diabetologists (ABCD) Nationwide Audit. Diabetes Care. 2020;43(9):2153-2160. doi:10.2337/dc20-0738
- Advancing Glycemic Management in People with Diabetes IQVIA 2019. Available at https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/advancing-glycemic-management-in-people-with-diabetes (Accessed on 28 11 2023)
- Beck RW, Bergenstal RM, Cheng P, et al. The Relationships Between Time in Range, Hyperglycemia Metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614-626. doi:10.1177/1932296818822496
- Beck RW, Riddlesworth T, Ruedy K, et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA. 2017;317(4):371-378. doi:10.1001/jama.2016.19975
- Visser MM, Charleer S, Fieuws S, et al. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6-month, prospective, multicentre, randomised controlled trial. Lancet. 2021;397(10291):2275-2283. doi:10.1016/S0140-6736(21)00789-3
- Choudhary P, Kolassa R, Keuthage W, et al. Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study [published correction appears in Lancet Diabetes Endocrinol. 2023 Jul;11(7):e9]. Lancet Diabetes Endocrinol. 2022;10(10):720-731. doi:10.1016/S2213-8587(22)00212-1
- Skyler JS. Diabetic complications. The importance of glucose control. Endocrinol Metab Clin North Am. 1996;25(2):243-254. doi:10.1016/s0889-8529(05)70323-6
- Jendle J, Buompensiere MI, Ozdemir Saltik AZ, et al. A European Cost-Utility Analysis of the MiniMed™ 780G Advanced Hybrid Closed-Loop System Versus Intermittently Scanned Continuous Glucose Monitoring with Multiple Daily Insulin Injections in People Living with Type 1 Diabetes. Diabetes Technol Ther. 2023;25(12):864-876. doi:10.1089/dia.2023.0297
- Ozdemir Saltik AZ, Yu J, Buompensiere MI, et al. EE622 Improved Glycemic Outcomes and Associated Cost Savings with an Advanced Hybrid Closed Loop System for People with Type 1 Diabetes with Suboptimal Glycemic Control in Europe. Value in Health. 2023;26(12):S172-S173. doi.org/10.1016/j.jval.2023.09.887
- International Diabetes Federation. IDF Diabetes Atlas, 9th edition. Brussels, Belgium. 2019. Available at: https://www.diabetesatlas.org
* When the recommended optimal settings of “the combination of glucose target setting at 100 mg/dL (5.5 mmol/L) and Active Insulin Time (AIT) at 2 hours” is used for at least 95% of the time. SmartGuard™ feature settings for each single patient must be defined by healthcare professionals based on individual targets and specific needs. The size of the cohort consistently using optimal settings was 6531 users in the study.
† Glucose Management Indicator (GMI) based on reported mean glucose values. Calculated using JAEB https://www.jaeb.org/gmi/.
‡ Compared to standard of care: Multiple Daily Insulin Injection (MDI) with Intermittently scanned continuous glucose monitoring (is-CGM), for the countries included in the analysis assuming a time horizon of 40 years. The MiniMed™ 780G insulin pump is indicated for use by patients aged 7-80 years with Type 1 diabetes.
§ Based on the IQVIA Core Diabetes Model simulation results, with clinical inputs derived from the ADAPT trial10.
** Compared to standard of care: Multiple Daily Insulin Injection (MDI) with Intermittently scanned continuous glucose monitoring (is-CGM), for the countries included in the analysis assuming a time horizon of 5 years.
The MiniMed™ 780G insulin pump is indicated for use by patients aged 7-80 years with Type 1 diabetes
- 1.ADA Guidelines of A1C<7% - ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic Targets: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- 2.International Consensus on TIR>70% - Battelino T, Danne T, Bergenstal RM, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. doi:10.2337/dci19-0028
- 3.Choudhary P, Arrieta A, van den Heuvel T, Castañeda J, Smaniotto V, Cohen O. Celebrating the Data from 100,000 Real-World Users of the MiniMed™ 780G System in Europe, Middle East, and Africa Collected Over 3 Years: From Data to Clinical Evidence. Diabetes Technol Ther. 2024;26(S3):32-37. doi:10.1089/dia.2023.0433
- 4.Carlson AL, Sherr JL, Shulman DI, et al. Safety and Glycemic Outcomes During the MiniMed™ Advanced Hybrid Closed-Loop System Pivotal Trial in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther. 2022;24(3):178-189. doi:10.1089/dia.2021.0319
- 5.Deshmukh H, Wilmot EG, Gregory R, et al. Effect of Flash Glucose Monitoring on Glycemic Control, Hypoglycemia, Diabetes-Related Distress, and Resource Utilization in the Association of British Clinical Diabetologists (ABCD) Nationwide Audit. Diabetes Care. 2020;43(9):2153-2160. doi:10.2337/dc20-0738
- 8.Beck RW, Riddlesworth T, Ruedy K, et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA. 2017;317(4):371-378. doi:10.1001/jama.2016.19975
- 9.Visser MM, Charleer S, Fieuws S, et al. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6-month, prospective, multicentre, randomised controlled trial. Lancet. 2021;397(10291):2275-2283. doi:10.1016/S0140-6736(21)00789-3
- 10.Choudhary P, Kolassa R, Keuthage W, et al. Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study [published correction appears in Lancet Diabetes Endocrinol. 2023 Jul;11(7):e9]. Lancet Diabetes Endocrinol. 2022;10(10):720-731. doi:10.1016/S2213-8587(22)00212-1
- 11.Skyler JS. Diabetic complications. The importance of glucose control. Endocrinol Metab Clin North Am. 1996;25(2):243-254. doi:10.1016/s0889-8529(05)70323-6
- 12.Jendle J, Buompensiere MI, Ozdemir Saltik AZ, et al. A European Cost-Utility Analysis of the MiniMed™ 780G Advanced Hybrid Closed-Loop System Versus Intermittently Scanned Continuous Glucose Monitoring with Multiple Daily Insulin Injections in People Living with Type 1 Diabetes. Diabetes Technol Ther. 2023;25(12):864-876. doi:10.1089/dia.2023.0297
- 13.Ozdemir Saltik AZ, Yu J, Buompensiere MI, et al. EE622 Improved Glycemic Outcomes and Associated Cost Savings with an Advanced Hybrid Closed Loop System for People with Type 1 Diabetes with Suboptimal Glycemic Control in Europe. Value in Health. 2023;26(12):S172-S173. doi.org/10.1016/j.jval.2023.09.887
- 14.International Diabetes Federation. IDF Diabetes Atlas, 9th edition. Brussels, Belgium. 2019. Available at: https://www.diabetesatlas.org
- 1.ADA Guidelines of A1C<7% - ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic Targets: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- 2.International Consensus on TIR>70% - Battelino T, Danne T, Bergenstal RM, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. doi:10.2337/dci19-0028
- 3.Choudhary P, Arrieta A, van den Heuvel T, Castañeda J, Smaniotto V, Cohen O. Celebrating the Data from 100,000 Real-World Users of the MiniMed™ 780G System in Europe, Middle East, and Africa Collected Over 3 Years: From Data to Clinical Evidence. Diabetes Technol Ther. 2024;26(S3):32-37. doi:10.1089/dia.2023.0433
- 4.Carlson AL, Sherr JL, Shulman DI, et al. Safety and Glycemic Outcomes During the MiniMed™ Advanced Hybrid Closed-Loop System Pivotal Trial in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther. 2022;24(3):178-189. doi:10.1089/dia.2021.0319
- 3.Choudhary P, Arrieta A, van den Heuvel T, Castañeda J, Smaniotto V, Cohen O. Celebrating the Data from 100,000 Real-World Users of the MiniMed™ 780G System in Europe, Middle East, and Africa Collected Over 3 Years: From Data to Clinical Evidence. Diabetes Technol Ther. 2024;26(S3):32-37. doi:10.1089/dia.2023.0433
- 10.Choudhary P, Kolassa R, Keuthage W, et al. Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study [published correction appears in Lancet Diabetes Endocrinol. 2023 Jul;11(7):e9]. Lancet Diabetes Endocrinol. 2022;10(10):720-731. doi:10.1016/S2213-8587(22)00212-1
- 6.Advancing Glycemic Management in People with Diabetes IQVIA 2019. Available at https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/advancing-glycemic-management-in-people-with-diabetes (Accessed on 28 11 2023)
- 7.Beck RW, Bergenstal RM, Cheng P, et al. The Relationships Between Time in Range, Hyperglycemia Metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614-626. doi:10.1177/1932296818822496
- 1.ADA Guidelines of A1C<7% - ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic Targets: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- 4.Carlson AL, Sherr JL, Shulman DI, et al. Safety and Glycemic Outcomes During the MiniMed™ Advanced Hybrid Closed-Loop System Pivotal Trial in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther. 2022;24(3):178-189. doi:10.1089/dia.2021.0319
- 5.Deshmukh H, Wilmot EG, Gregory R, et al. Effect of Flash Glucose Monitoring on Glycemic Control, Hypoglycemia, Diabetes-Related Distress, and Resource Utilization in the Association of British Clinical Diabetologists (ABCD) Nationwide Audit. Diabetes Care. 2020;43(9):2153-2160. doi:10.2337/dc20-0738
- 1.ADA Guidelines of A1C<7% - ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic Targets: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97-S110. doi:10.2337/dc23-S006
- 2.International Consensus on TIR>70% - Battelino T, Danne T, Bergenstal RM, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. doi:10.2337/dci19-0028
- 3.Choudhary P, Arrieta A, van den Heuvel T, Castañeda J, Smaniotto V, Cohen O. Celebrating the Data from 100,000 Real-World Users of the MiniMed™ 780G System in Europe, Middle East, and Africa Collected Over 3 Years: From Data to Clinical Evidence. Diabetes Technol Ther. 2024;26(S3):32-37. doi:10.1089/dia.2023.0433
- 11.Skyler JS. Diabetic complications. The importance of glucose control. Endocrinol Metab Clin North Am. 1996;25(2):243-254. doi:10.1016/s0889-8529(05)70323-6
- 12.Jendle J, Buompensiere MI, Ozdemir Saltik AZ, et al. A European Cost-Utility Analysis of the MiniMed™ 780G Advanced Hybrid Closed-Loop System Versus Intermittently Scanned Continuous Glucose Monitoring with Multiple Daily Insulin Injections in People Living with Type 1 Diabetes. Diabetes Technol Ther. 2023;25(12):864-876. doi:10.1089/dia.2023.0297
- 12.Jendle J, Buompensiere MI, Ozdemir Saltik AZ, et al. A European Cost-Utility Analysis of the MiniMed™ 780G Advanced Hybrid Closed-Loop System Versus Intermittently Scanned Continuous Glucose Monitoring with Multiple Daily Insulin Injections in People Living with Type 1 Diabetes. Diabetes Technol Ther. 2023;25(12):864-876. doi:10.1089/dia.2023.0297
- 13.Ozdemir Saltik AZ, Yu J, Buompensiere MI, et al. EE622 Improved Glycemic Outcomes and Associated Cost Savings with an Advanced Hybrid Closed Loop System for People with Type 1 Diabetes with Suboptimal Glycemic Control in Europe. Value in Health. 2023;26(12):S172-S173. doi.org/10.1016/j.jval.2023.09.887
- * When the recommended optimal settings of “the combination of glucose target setting at 100 mg/dL (5.5 mmol/L) and Active Insulin Time (AIT) at 2 hours” is used for at least 95% of the time. SmartGuard™ feature settings for each single patient must be defined by healthcare professionals based on individual targets and specific needs.
The size of the cohort consistently using optimal settings was 6531 users in the study.
- † Glucose Management Indicator (GMI) based on reported mean glucose values. Calculated using JAEB https://www.jaeb.org/gmi/.
- ‡ Compared to standard of care: Multiple Daily Insulin Injection (MDI) with Intermittently scanned continuous glucose monitoring (is-CGM), for the countries included in the analysis assuming a time horizon of 40 years. The MiniMed™ 780G insulin pump is indicated for use by patients aged 7-80 years with Type 1 diabetes.
- § Based on the IQVIA Core Diabetes Model simulation results, with clinical inputs derived from the ADAPT trial10.
- ** Compared to standard of care: Multiple Daily Insulin Injection (MDI) with Intermittently scanned continuous glucose monitoring (is-CGM), for the countries included in the analysis assuming a time horizon of 5 years.
The MiniMed™ 780G insulin pump is indicated for use by patients aged 7-80 years with Type 1 diabetes
- Quality-adjusted life-year
- * When the recommended optimal settings of “the combination of glucose target setting at 100 mg/dL (5.5 mmol/L) and Active Insulin Time (AIT) at 2 hours” is used for at least 95% of the time. SmartGuard™ feature settings for each single patient must be defined by healthcare professionals based on individual targets and specific needs.
The size of the cohort consistently using optimal settings was 6531 users in the study. - † Glucose Management Indicator (GMI) based on reported mean glucose values. Calculated using JAEB https://www.jaeb.org/gmi/.
- ‡ Compared to standard of care: Multiple Daily Insulin Injection (MDI) with Intermittently scanned continuous glucose monitoring (is-CGM), for the countries included in the analysis assuming a time horizon of 40 years. The MiniMed™ 780G insulin pump is indicated for use by patients aged 7-80 years with Type 1 diabetes.
- § Based on the IQVIA Core Diabetes Model simulation results, with clinical inputs derived from the ADAPT trial10.